Designed & Developed by Technocrat Software
Quality Assurance in Sterilization – SPD India’s Full Range of CSSD Consumables & Cleaning Chemicals
At SPD India Healthcare Pvt Ltd, we are committed to advancing patient safety by offering a comprehensive portfolio of consumables and cleaning agents that ensure not just sterilization—but verified sterilization. Our range includes indicators, test systems, packaging materials, and instrument cleaning solutions, all of which are essential for achieving and maintaining the highest standards in infection control. Whether you're validating a sterilization cycle or preparing instruments for reprocessing, SPD India provides proven, compliant, and easy-to-use solutions.
Comprehensive Range Of Maintenance Services
Comprehensive Range Of Maintenance Services

Chemical Indicators (CI) – Types 1 to 6
Purpose: Visual verification of sterilization parameters Chemical indicators visually confirm that critical parameters (temperature, time, sterilant presence) have been achieved during sterilization.
Type 1:
External indicators on pouches/packs.
Type 2:
Bowie-Dick tests for air removal (pre-vacuum).
Types 4 & 5:
Multi-variable indicators for internal pack monitoring.
Type 6:
Emulating indicators offering precise verification.

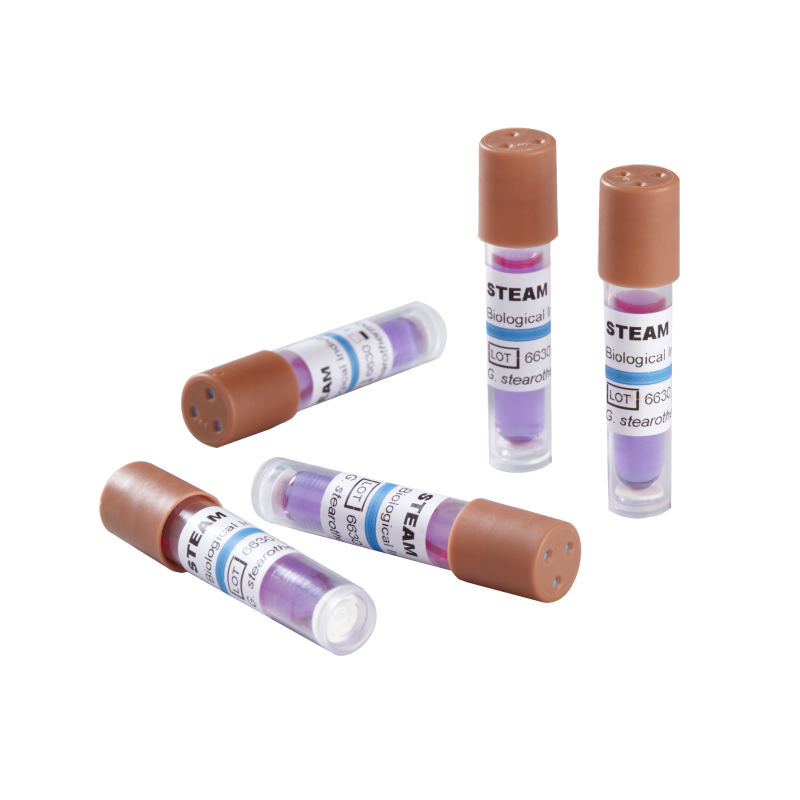
Biological Indicators (BI)
Purpose: Gold standard for sterilization validation Contain resistant spores (e.g., Geobacillus stearothermophilus) to verify sterilizer lethality. Available in self-contained vials or strips for:
Steam Sterilization
Ethylene Oxide (EO)
Hydrogen Peroxide (Plasma)

Bowie-Dick Test Pack
Purpose: Daily test for air removal and steam penetration in pre-vacuum sterilizers Detects the presence of residual air or vacuum pump failures. Ensures consistent sterilization cycle performance.

Sterilization Indicator Tapes
Purpose: Sealing packs with visible sterilization confirmation Available for Steam, EO, and Plasma cycles, with clear color-change indicators and strong adhesion.

Sterilization Pouches & Reels
Purpose: Maintain sterility post-processing Constructed from medical-grade paper and multilayer film, with integrated indicators. Available in various sizes for surgical sets, instruments, and kits.
| Serial No. | Product Code | Size |
|---|---|---|
| 1 | SPD/ PP/ 002 | 75 mm X 200 mt |
| 2 | SPD/ PP/ 003 | 100 mm X 200 mt |
| 3 | SPD/ PP/ 004 | 150 mm X 200 mt |
| 4 | SPD/ PP/ 005 | 200 mm X 200 mt |
| 5 | SPD/ PP/ 006 | 250 mm X 200 mt |
| 6 | SPD/ PP/ 007 | 300 mm X 200 mt |
| 7 | SPD/ PP/ 008 | 350 mm X 200 mt |
| 8 | SPD/ PP/ 009 | 400 mm X 200 mt |
| 9 | SPD/ PP/ 010 | 450 mm X 200 mt |

Internal Indicator Strips & Cards
Purpose: Confirm sterilant penetration inside packs. Placed deep within trays or packs to ensure full exposure, particularly for complex instruments or large sets.
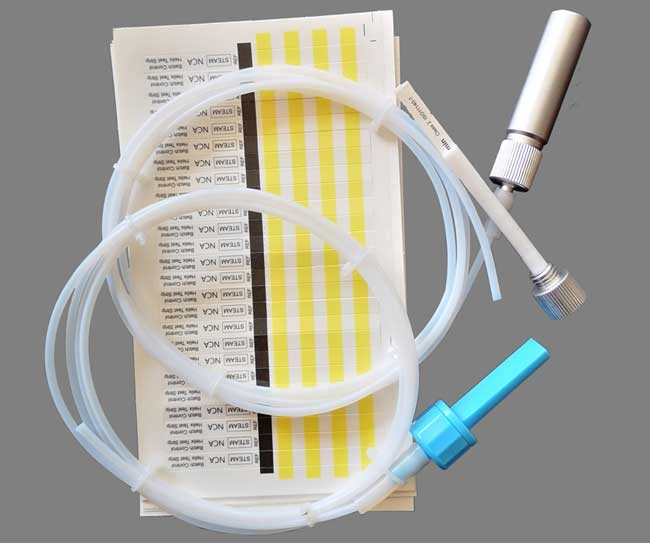
Helix Test Device (PCD)
Purpose: Simulates hollow loads in Class B sterilizers Used with Type 6 indicators to test steam penetration in instruments with long, narrow lumens (e.g., endoscopes, laparoscopic tools).
EO & Plasma Sterilization Indicators
Purpose: Monitoring low-temperature sterilization Available in both chemical and biological formats. Ensures safe and effective reprocessing of heat-sensitive items.

Record-Keeping & Log Books
Purpose: Traceability and compliance Includes sterilization logbooks, BI result sheets, and equipment maintenance records to meet NABH and international standards.

Sterilization Process Audits
Purpose: Ensuring adherence to protocols and improving sterilization efficiency. Audits provide insights into compliance and help identify gaps in practices.
Protocol Adherence
Auditors verify if standard sterilization procedures are being followed. Deviations are documented for corrective action.
- Review standard operating procedures (SOPs).
- Observe actual practices in the CSSD.
Equipment Validation
Assessment of sterilizer functionality and calibration. Ensures machines are operating within required parameters.
- Check autoclave calibration certificates.
- Test cycle consistency using indicators.
Staff Competency
Evaluation of staff knowledge on sterilization techniques. Training needs are identified based on performance gaps.
- Conduct staff interviews or quizzes.
- Review training records and certifications.
Record Review
Inspection of logbooks, BI results, and maintenance reports. Accurate documentation ensures traceability and legal compliance.
- Examine BI incubation records.
- Verify completeness of maintenance logs.
Why SPD India?
- Complete end-to-end solution for CSSD quality assurance.
- Products conform to ISO 11140, ISO 11138, CE, NABH, and WHO standards.
- Trusted by leading hospitals, medical colleges, and private institutions.
- Backed by expert technical support and onsite training.
Partner with SPD India
Let us help you build a world-class CSSD. With our range of sterilization verification products and cleaning solutions, you’re not just sterilizing—you’re ensuring, documenting, and protecting at every stage.